Data sharing
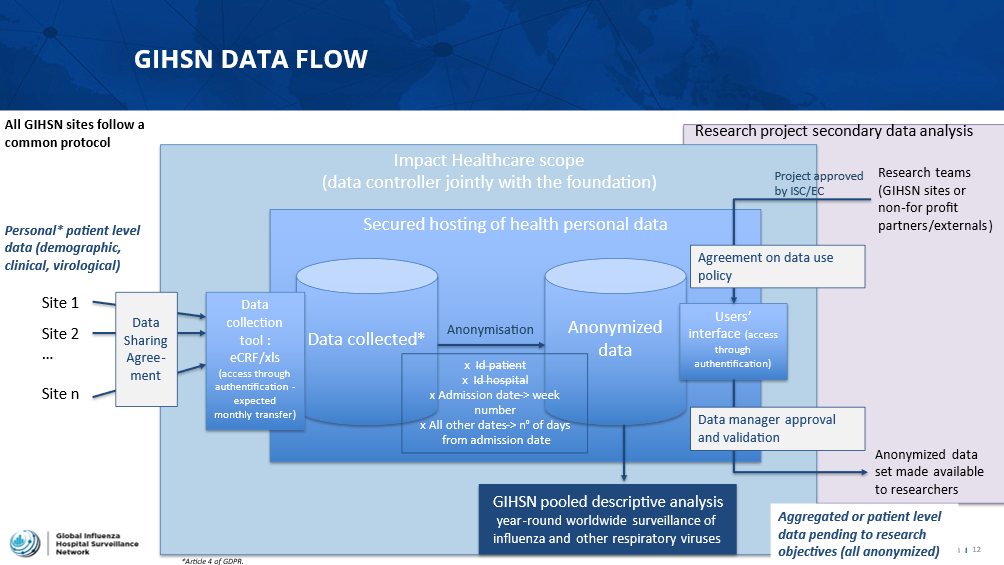
To comply with data access and privacy regulations, the Foundation set up a data warehouse and a data access framework. Impact Healthcare is the Data Controller for the GIHSN (jointly with Fondation de France), handling the data collection process and overseeing the GIHSN data warehouse. The GIHSN database is hosted in a secure environment (certified secured hosting for health personal data).
Data is processed in full accordance with the European General Data Protection Regulation (GDPR) and French data protection regulations.
Data collected by sites receiving funding remains the proprietary of the site. There is no commercial use of the data. Donors of the Foundation for Influenza Epidemiology do not have access to the data.
A data sharing agreementis signed by each site before field implementation starts. Sites implementing the GIHSN protocol should be compliant with their ethical and national regulations for the conducting of the surveillance. Any obligation related to data protection and data transfer to the GIHSN platform should be anticipated.
Download data sharing agreement
Data analysis
A yearly pooled analysis is proposed, describing the season, or combining data from various sites and/or years for pooled analyses. A yearly manuscript is developed under the responsibility of the Independent Scientific Committee.
Additional analyses can be performed by research teams after review and approval of their research proposal by the Independent Scientific Committee and the Foundation. Data access (anonymised and/or aggregated data only) is granted to research teams through a dedicated interface. The data catalogue is available on the platform, together with sites description. This provides a high-level fingerprinting of the GIHSN database and allows researchers to assess the feasibility of their research question.
Sites are informed upfront of any analysis, and they have the possibility to opt out.
Ethics
The GIHSN design and protocol has to be approved by local Research Ethics Committee before field implementation starts. Informed written consent may be required for enrolment. No intervention, apart respiratory specimens (e.g., nasopharyngeal, nasal and oropharyngeal) is associated with the study.